Recent Progress and Future Challenges of Functional Textiles Fabricated by Atomic Layer Deposition- Juniper Publishers
Journal of Fashion Technology- Juniper publishers
Abstract
Atomic Layer Deposition (ALD) technique has been regarded as one of the most efficient and promising approaches for the functionalization of complex-shaped surfaces due to its precise and simple thickness control, excellent conformality. Additionally, mild growth conditions such as low temperature and oxygen-free characteristic of ALD made it particularly attracting for the fabrication of functional textiles. Functionalization of natural or synthetic fabrics such as cotton, silk, polyvinyl alcohol, Kevlar and even to chemical-inert Carbon Fiber (CFF) via ALD to endow them with super hydrophobicity, conductivity, antibacterial activity, UV-blocking property, anti-yellowing property, and retardancy are reviewed. Finally, the challenges and perspectives on the application of ALD in the functionalization of fabrics are proposed.
Keywords: Atomic layer deposition; Functional fabrics; Future challenges
Introduction
The natural and synthetic fibers have been deeply rooted into our daily life and it is unimaginable that the life without fabrics. Increasing damage caused by exposure to microbes, chemical, pesticide, UV light and pollutants has strengthened the demand for fabrics which are expected to be waterproof [1], flame retardant, antimicrobial [2-3], self-cleaning [1,4-5] and so on. Conventional finishing methods such as pad-dry-cure or coating are widely used to impart desired functions feather to fabrics in industry. While the excessive add-on weight, poor washing stability, weaken mechanical strength and reduced comfort to wearer are eagerly to be solved. Alternatively technologies, which are eco friendly, laundering durable [1], cost effective and do not lower the comfortability while maintaining optimum protection functions are still being eagerly pursued.
Many new functionalization methods and techniques, including enzyme immobilization [6], layer by layer assembling [7], electrophoretic deposition [8], and Atomic Layer Deposition (ALD) [9-13] are utilized to achieve the multi-functionalization of fabrics. Among them, ALD technique has been regarded as one of the most efficient and promising approaches due to its precise thickness control down to atomic level, excellent conformality and capability for deposition onto the complex-shaped surfaces. Additionally, the ALD of metal oxides such as Al2O3, TiO2 and ZnO could even be carried out at low temperature, which render great interest in the surface-modification for temperature-sensitive fabrics.
ALD is realized by repeating cycles of self-limiting gas-solid reactions adhere to self-assembly chemistry. The precursor gases are alternately pulsed into the reactor, and excess gaseous precursors and by-products are removed by an inert purge gas pulse after each half-reaction. For each cycle, only one atomic layer is formed on the surface, enabling tailored thickness down to atomic/molecular level on ideal/complex surface. Desired thickness could be easily achieved by repeating the ALD cycles to a certain number. The advantages in the precisely thickness controlling and resulted excellent conformal thin film made ALD a good candidate for applications in the functionalized fabrics [14].
Hence, a review covers both of the growth mechanism and applications of ALD on fiber/fabrics is necessary to clarify their recent progress. In this review, the general description of ALD growth mechanism was firstly introduced briefly. Then the extended mechanism is proposed to understand the surface growth mechanism of ALD on fiber/fabrics. Finally, the future challenges of applications in ALD-modified functional fabrics is presented.
ALD Fundamentals
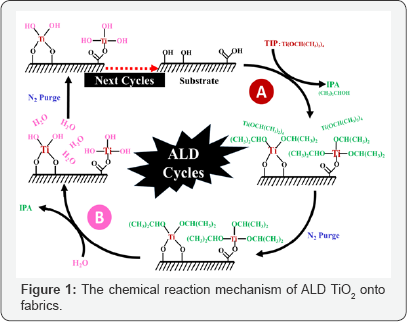
Figure 1 depicted the typical reaction mechanism of the low-temperature ALD process with Titanium (IV) IsoPropoxide (TIP)/water (H2O) for ALD deposition of TiO2 onto fiber/fabrics. Firstly, the TIP moleculars were pulsed and adsorbed onto the substrate surface and react with active hydroxyl group or carboxyl group to form a monolayer TiO2 layer. By-products and unreacted precursor were purged out by followed pulse of high- purity nitrogen (N2). By repeating above-mentioned cycle to certain numbers, the TiO2 layers with desired coating thickness are obtained, indicating that the growth mode is generally close to 2D mode.
In fact, one ALD cycle consists of two time-separated half reactions. In the first half reaction, the substrate is exposed to TIP vapor that forms a sub-monolayer films of the precursor on the surface of the substrate, followed by the use of a purge gas to remove the excess TIP precursor. In the second half reaction, the subsequently pulsed reactant gas reacts with the as-adsorbed precursor layer to form a target film. After such cycle, only one TiO2 molecular layer is deposited onto the surface of the substrate.
Applications of ALD technique in the functionalization of fabric
Different with the surfaces of inorganic solids such as silicon wafer, woven and nonwoven fabrics normally made from natural or synthetic polymer suffered from the lacking of surface-bound active groups such as hydroxyl groups which are indispensable to initiate the ALD reaction. This makes the woven and nonwoven fabrics to adopt a very different growth mechanism. Firstly, the first reaction between precursor and polymeric fabric surface always accompanied by a precursor molecular penetrating process. In this case, several different kinds of chemical bonds will form between the inorganic nanoparticles layer and polymeric fabrics compared with that on inorganic solid surface. Secondly, numerous inter-chain gaps at different sizes allow the penetration of ALD precursors inside the bulk of fabrics which results in different surface morphology and roughness. Not only the outmost exposed surface but also the interior surface of polymer chains could be deposited. Thirdly, other active groups involved in the polymer frame such as amide, carboxyl, hydroxyl group provide more complicated chemical reactions leading to various reaction products and mechanisms. The reaction mechanisms of ALD onto polymeric fabrics could be classified into two types: (i) polymeric fabrics with abundant surface- bound reactive groups, particularly hydroxyl groups, and (ii) fabrics lacking of surface reactive groups.
Polymeric fabrics with copious reactive groups
Some fabrics such as cotton, silk, wool, cellulose, polyvinyl alcohol, polyamide are kind of fabrics with abundant surface- bound active groups including -OH, -COOH, -NH2, which are widely accepted as effective groups to form covalent bond with ALD inorganic layers (ZnO, TiO2, Al2O3 and so on). The multiple functions properties, such as antibiosis, UV-blocking [15], antioxidation [16-18], superhydrophobicity [13,19], conductivity [11,20-31], and retardancy [32,33] could be endowed to polymeric fabrics after ALD of different inorganic layers onto their surface.
Regulating wettability of fabrics by ALD: The desire for fabrics with self-cleaning property provides strong motivation to the research on how to regulate surface wettability via ALD. The wettability of water on textured surfaces depends on both the surface topography (tightly linked with surface roughness) and the surface energy of the fabric. By selected exposing outmost function group after ALD, the surface can be switched between hydrophobic and hydrophilic. Upon coating by ALD Al2O3, nonwetting polypropylene surface was switched to a water wetting state while cotton fabrics surface was switched from a fully wettable state to a strong hydrophobic state for a long time even after one cycle of Tri Methyl Aluminum (TMA) deposition. This difference was attributed to the more stable Al-(O-CH3)3-n groups and few unstable hydrophilic Al-OH groups on the outmost surface of cellulose fabric [13]. A carbon absorption after deposition also contributed to this long-lasting hydrophobicity. Another work by Hyde [9] reveals that cotton textiles modified with conformal nanoscale Al2O3 are found to show extreme hydrophobic effects, distinctly different from planar surfaces that receive the same coatings. This convinced that the extreme hydrophobicity (contact angle increases from 0 to 127o) lies on both the low surface energy and the surface roughness. This regulation of the surface wettability via ALD was furtherly applied to improve the print quality of wet-laid nonwoven cellulose fabric by coating TiO2 and Al2O3 layers using titanium tetrachloride/H2O and TMA/H2O as precursors, respectively [24]. To some extremely case, even a superhydrophobic surface with a water contact angle up to 160° could be successfully constructed on the wool fabric by ALD Al2O3 [13].
Conductive coatings on textiles: Conductive fibers are excellent candidates for a broad range of applications, including chemical sensing, energy storage, flexible antennas, and foldable displays. More importantly, metal ALD coatings on fabrics provides solvent-free functionalization of textiles for electronic applications. Sweet [11] tried to improve the conductivity of the nonwoven polypropylene fabric by coating ZnO layer onto their surfaces but it doesn't work. The transmission electron microscopy shows that the conductive ZnO penetrates into the porous fabric and thus contribute few to the conductivity. Inserting a insulated ALD Al2O3 layer prior to the ZnO growth could effectively block the penetration of the ZnO gaseous reactants into the fabrics thusly resulted a higher effective conductivity (> 25 Scm-1) after 200 ALD ZnO cycles. For comparison, ZnO deposition on nonwoven nylon-6 fabric exhibits uniform growth without Al2O3 pretreatment and the conductivity again changes significantly with the Al2O3 pretreatment. Jur et al. [28] also reported the coating of cotton fibers with ZnO via ALD to introduce the conductivity. The conductivity of coated cotton fibers increases with the increase in coating thickness. Different with using metal oxide coatings, low-temperature vapor- phase tungsten Atomic Layer Deposition (ALD) using WF6 and dilute silane (SiH4, 2% in Ar) yields highly conductive coatings on nylon-6 micro fiber mats, producing flexible and supple nonwovens with conductivity of ~1000 S/cm [29]. Mundy et al. [21] also produce highly conductive nonwoven nylon-6 fiber mats having effective conductivities as high as 5500-6000 S/cm with only a 6% fractional increase in mass by low temperature platinum atomic layer deposition via (methylcyclopentadienyl) trimethyl platinum and ozone.
UV-blocking textiles: UV irradiation can cause a severe damage to textiles in terms of color, mechanical strength, and human physiological comfort. Inorganic UV absorbers like ZnO, SiO2, and TiO2 in the micro- or nanoscale are in particularly attractive due to their superior performance. Xiao et al. [15] deposited TiO2, Al2O3, and TiO2/Al2O3 nano-layer onto dyed polyamide/aramid blend fabric surface. The dyed fabrics after ALD coating showed excellent UV resistance and high resistant to UV-induced mechanical strength damage.
Apart from synthetic polymer fabrics as mentioned above, natural polymer fabrics such as silk fabrics showed much better UV-blocking properties after coated with TiO2 by ALD. The strong absorbance against UV of TiO2 made it a good UV sacrificial absorbent which could significantly protect the silk fiber from UV-induced yellowing and deterioration in mechanical property [16].
Fabrics lacking of reactive groups-Carbon fibers or fabrics
Different with polymeric fabrics with copious surface- bound reactive groups, carbon-based fabrics are short of active groups which can't initiate the ALD reaction. While surface pretreatment to carbon fiber fabrics are widely adopted in order to the increase their interface binding force with epoxy. This pretreatment provide the possibility for the ALD by more or less generating some active groups on the surface of carbon fiber fabrics. The amount of these active groups are sufficient to form a dense inorganic layer on the fabrics via ALD. For other fabrics short of surface-bound active groups, plasma treatment under oxygen is a promising method for introducing active groups.

Coloration of dye-inert fabrics: As a high-performance fibers, carbon fibers play a very important role in a variety of hightech fields, including aerospace, sports, automotive, chemical industry, infrastructure, military, energy, reinforcement in composite materials, textiles, and semiconductors, owing to their remarkable properties, such as high strength, stiffness, heat and chemical resistance, low densities, good thermal and electrical conductivities, excellent creep resistance, biological compatibility, and fatigue resistance [34-37]. However, coloring of carbon fibers is difficult due to insufficient chemical affinity between carbon fibers and dyes and their high crystallinity. Multicolored carbon fiber fabrics was recently prepared by Chen et al. using ALD, which showing various and vibrant structural colors [38] by controlling the thickness of coated conformal TiO2 layer while only slightly weaken their mechanical properties Figure 2.
Improvement of anti-oxidation property of fabrics: Meanwhile, carbon fibers are sensitive to oxidation and tensile properties will deteriorate strongly when temperatures exceeding 400 oC in oxidizing humid conditions [39-42]. To protect carbon fibers from reaction with oxygen, coating a diffusion barrier made from silica, alumina, titania, etc onto fibers is a successful strategy. Westwood et al. [43], coated alumina onto carbon fibers by ALD via sequential exposed to tri methyl aluminum and water at the low temperature (~77 oC). The starting oxidation temperature of carbon fiber fabrics was significantly increase from 300 oC (pristine carbon fiber fabrics) to 600 oC (alumina layer with the thickness of 30nm) and 660 oC (alumina layer with the thickness of 120 nm) respectively.
Challenges and outlooks
In this review, we summarized the reaction mechanism and surface chemistry of ALD deposition on fabrics as well as the existing functionalizations of natural and synthetic fabrics via ALD. The ability of ALD to regulate the surface energy, reactivity and wettability of polymeric fabrics widens its application in separations, electronic-chemical system, biological scaffolds, energy conversion devices, and flexible chemically active systems.
Despite the achievements made over recent years, many challenges still remain for the future development of ALD in fabrics, such as (i) difficulties in theoretical modeling of the reactions on sophisticated fabric surface, (ii) difficulties in scaling up the ALD to large volume for industry production, (iii) reaching an acceptable process speed. A system and method for continuous atomic layer deposition was patented by Elam [23]. This atmosphere applicable ALD system which can work continuously expands the further application of ALD for the roll-to-roll production of functional fabrics. We believe that all existing researches on ALD modified fabrics would open a new doors in the market for functional fabrics particularly when combined with the well-developed roll-to-roll technology.
Acknowledgement
This research was financial supported from the National Science Foundation for Distinguished Young Scholars (Grant No. 51325306) and the National Natural Science Foundation of China (Grant No. 51773158). The authors would like to acknowledge the Key Laboratory of Textile Fiber & Product (Wuhan Textile University) Grant (Project no. FZXW2017013) for support of this project. Fengxiang Chen and Xin Liu contributed equally to this works.
To know more about Journal of Fashion Technology-https://juniperpublishers.com/ctftte/index.php
To know more about open access journals Publishers click on Juniper Publishers




Comments
Post a Comment